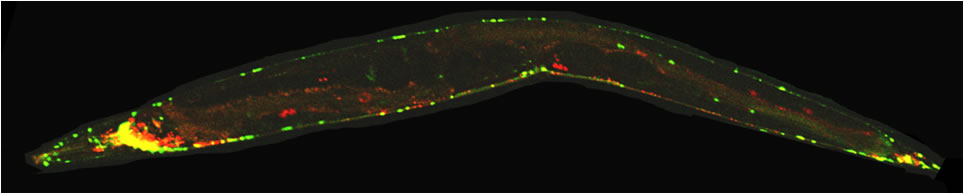
Institute of Molecular and Cellular Biology & Department of Life Sciences
Dr. Oliver Wagner, Professor
Current research interests
(1) Regulation of axonal transport in the nervous system of C. elegans
(a) Regulation of molecular motors: How does cargo bind to motors and how does cargo-binding affect motor motility?
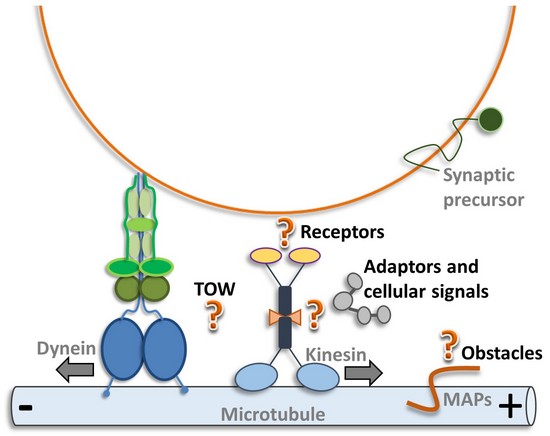
(b) Bidirectional transport: What is the basis of frequently observed directional changes of synaptic vesicles and mitochondria in axons (TOW, tug-of-war, or activity coordination)?
(c) Obstacles on the highway: How do motors, synaptic vesicles and mitochondria find their way thru the crowded axon? Do the various microtubule-binding proteins (MAPs, tau, plectin etc.) or neurofilaments affect their motility?
(d) What is the role of post-transalational modification of tubulin on neuronal transport?
(2) Cilia development and intraflagellar transport
(a) Which kinases and phosphatases affect C. elegans cilium biogenesis and intraflagellar transport?
(b) What is the role of post-transalational modification of tubulin on intraflagellar transport?
Approaches
(1) Regulation of axonal transport in the nervous system of C. elegans
Neuronal cells are composed of a cell body and long cytoplasmic processes termed dendrites and axons. In the neuronal cell body proteins are synthesized, packed in vesicles and transported long ranges thru the axon and dendrites to the synapses. The neuronal processes are, however, overloaded with vesicles, synaptic precursors, molecular motors and cytoskeletal elements. Thus, it is not surprising that defects in the motor-cargo transport system can lead to abnormal aggregation of disease proteins and concomitant neuronal cell death. It is remarkable that many of these disease-related proteins have been identified as motor-interacting proteins or proteins present in cargo. For example Alzheimer's disease occurs due to the accumulation of amyloid precursor proteins which is a cargo for the molecular motor KIF5A. A mutation in the motor dynein rescues axonal transport defects and extends the life span of ALS (Amyotrophic lateral sclerosis) mice. LIS1 is associated with dynein while a mutation in LIS causes lissencephaly (severe mental retardation and poor control of movement). A mutation in the molecular motor KIF1Bb causes the human peripheral neuropathy Charcot-Marie-Tooth (CMT) disease type 2A (leading to severe muscle weakness, inability to perceive touch, pain and temperature changes). A single base-pair change in the p150 Glued subunit of the motor adaptor protein dynactin causes LMN (lower motor neuron disease). Genetic defects in KIF1A (termed UNC-104 in C. elegans) are linked to KIF1A-associated neurological disorders (KAND) encompassing hereditary spastic paraplegia (HSP), senile dementia and CMT.
Above examples indicate that proper function and regulation of the motor-cargo transport system is critical and that many neurodegenerative diseases display accumulation of cargo based on defects in molecular motors. However, the mechanisms of cargo transport and the regulation of motors on the molecular level is only partial known. One of the basic questions is how to explain the frequent observed bidirectional movement of molecular motors and vesicles. One common hypothesis is that one vesicle binds multiple types of motors and they either act in a cooperative manner or they compete with each other in a tug-of-war. In contrast to bidirectional movement of vesicles (on which surfaces can bind multiple cargos), the frequent observed bidirectional movement of single GFP-tagged motors might be explained by direct interaction of an opposing motor. One of our hypothesis is that bidirectional movement of anterograde (from the cell body to the synapses) moving kinesins can be explained by a direct and regulating interaction with retrograde moving dynein (from the synapses to the cell body) and that this regulating interaction may be mediated by the dynein-cargo adaptor protein dynactin.
a) Regulation of molecular motors
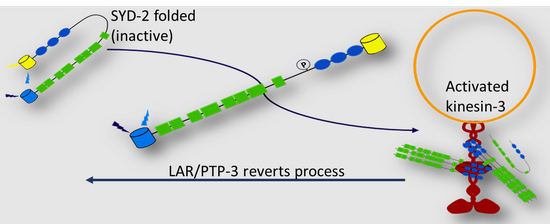
In our previous research we investigated the interaction between a major axonal transporter (kinesin-3 UNC-104/KIF1A) and one of its cargo SYD-2/liprin-a. SYD-2/liprin-a is a multifunctional active zone protein and important for synaptic function and synaptogenesis. Interestingly, SYD-2 scaffolds the motor UNC-104 in the nervous system of C. elegans probably making active motors available along the lengthy axons. We assume that inactive motors are exchanged by active motors at the sites of clustering. Still, SYD-2 acts bifunctional: Besides its scaffolding function we found that it regulates the motor's directionality and speed (Wagner et al., PNAS, 2009; Chia et al., Mol. Cell Neurosci., 2013). Interestingly, we identified another active zone protein LIN-2/MAGUK with similar functions to SYD-2 likely because these two proteins share a similar binding site on UNC-104. We have also shown that these two proteins act differentially on regulating UNC-104 when bound to specific region on the motor (specifically in regard on velocity and run length regulation) (Wu et al., Traffic, 2017). Recently, we reported that SYD-2 unfolds and becomes activated after phosphorylation at position Y741 to interact with UNC- 104 (Shanmugam et al., MBoC, 2020). Phosphatase LAR/PTP-3, a transmembrane receptor for SYD-2, is able to revert this process:
A recently published project focused on the effect of RIM (UNC-10) on SYD-2's binding properties to kinesin-3. It is known that RIM interacts with liprin-a and that this complex associates via RAB3 to the vesicle membrane. However, whether there is a regulating function of UNC-10 in the SYD-2/UNC-104 complex is still unknown. We found that the protein complex RAB3-UNC-10-SYD-2 acts as an additional link that facilitates UNC-104's interaction with the synaptic vesicle membrane (besides the UNC-104 PH-domain that is known to directly associated with the lipid-bilayer) (Bayansan et al., Neurobiol. Dis., 2025).
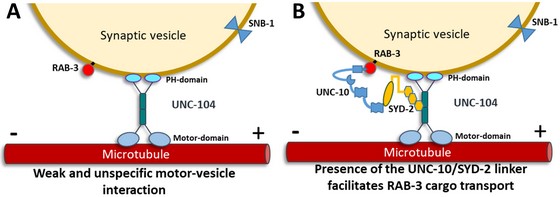
b) Bidirectional Transport
One key question in axonal transport is how molecular motors and synaptic vesicles undergo the frequent observed directional changes. As mentioned above, an idea would be that several (opposing) molecular motors bind to the vesicle membrane and act in a tug-of-war process. The other idea is whether direct and regulative interaction occur between opposing molecular motors as kinesins (anterograde motor) and dynein (retrograde motor). Indeed, we have recently shown direct UNC-104/dynein and UNC-104/dynactin interactions (Chen et al., J. Neurosci. Res., 2019):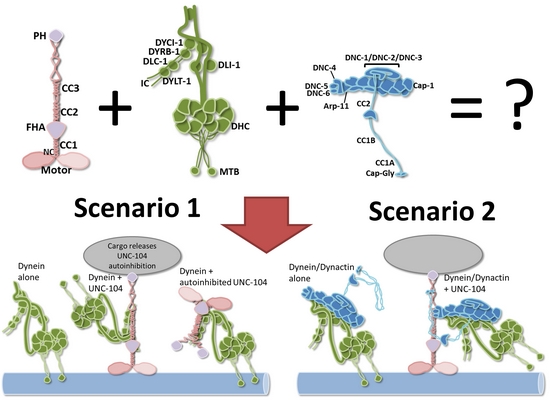
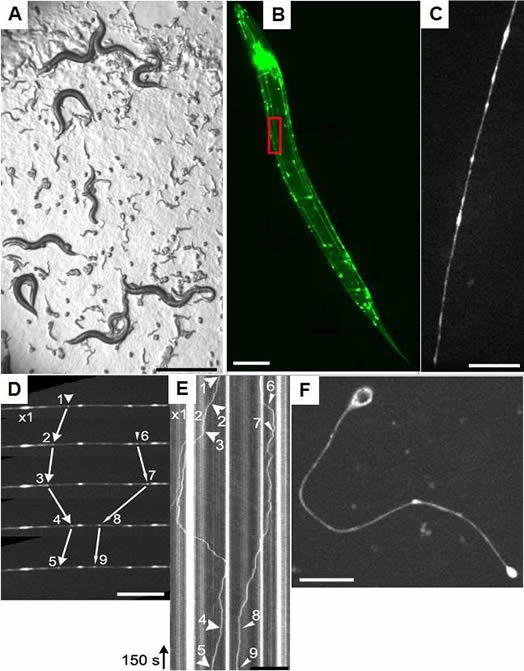
Critically, using C. elegans we are able to perform high-speed and high-resolution single motor molecule tracking in live animals with different mutant backgrounds. We are using a confocal spinning disk microscope that is connected to a high-sensitive EMCCD camera. An example of time-lapse imaging of UNC-104 in a single neuron of a living animal is shown below:
(A) C. elegans expressing a GFP-tagged molecular motor named UNC-104/KIF1A (brightfield photograph). C. elegans eggs and larvae are evident. (B) Fluorescent photograph of the worm expressing the GFP-tagged molecular motor UNC-104/KIF1A which is specifically expressed in the nervous system. (C) Magnification of inset in (B) displaying a single neuron and molecular motors inside the living worm. (D) Motility of UNC-104/KIF1A particles was analyzed using confocal time-lapse imaging. (E) Kymograph image from (D) making traces of the molecular motor in time and space evident. (F) Single isolated (primary) neuronal cell expressing the molecular motor UNC-104/KIF1A. Scale bars: (A) 1 mm, (B) 100 microns, (C+D) 10 microns, (E) 50 microns and (F) 10 microns. |
c) "Obstacles on the highway": Effect of microtubule-associated proteins and neurofilaments on axonal transport
Microtubule-associated proteins as, i.e., tau-protein play a major role in many neuropathological phenotypes (Alzheimer's, FTDP17) and it is of major interest whether accumulated cytoskeletal elements as tau (Alzheimer's) or neurofilaments (ALS) affect axonal transport. We are currently investigating if mutations or overexpression of tau, plectin (neurofilament and microtubule binding protein) as well as neurofilaments affect transport characteristics of synaptic vesicles or molecular motors. We have previously reported an interaction between the molecular motor dynein and neurofilaments (Wagner et al., Mol. Biol. Cell, 2005) as well as between a novel tau homolog PTL-1 in C. elegans and axonal transporter UNC-104/KIF1A (Tien et al., Neurobiol. Dis., 2011). Critically, have thoroughly characterized a new neurofilament-like protein TAG-63 in C. elegans (Bhan et al., Traffic, 2019). C. elegans can now be used as model organism to study neurofilament-based neurological diseases.
As we have reported earlier that neurofilaments functional associate with mitochondria (Wagner et al., J. Neurosci., 2003) we extended the idea whether intermediate filaments are involved in mitochondria transport in general. After screening a dozen of intermediate filament mutants to evaluate how they affect mitochondria movement in neurons, we identified IFB-1 to positively support transport of these organelles (Barmaver et al., 2022, Traffic).
(2) Cilia development and intraflagellar transport
Adult polycystic kidney disease (APKD) is one of the most common diseases in Taiwan and it is supposed to be linked to defects in the highly conserved components of the cilia and IFT (intraflagellar transport) machinery. Other ciliary disorders include primary ciliary dyskinesia (PCD), Bardet-Biedl syndrome (BBS), Meckel-Gruber syndrome (MKS) and nephronophthisis.
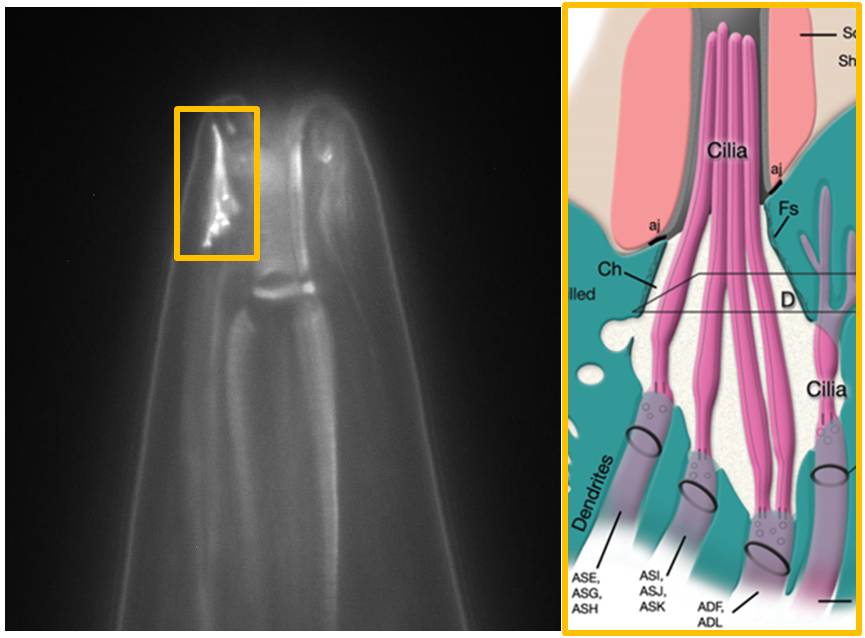 |
|
The image above shows the head ("nose") of a worm with stained amphid cilia. The transition zone, middle segment and distal segment are visible. The corresponding schematic drawing to the right shows the arrangement of the cilia - emanating from amphid dendrites - into a bundle. The diameter of the worm near the tip of the head is about 35 micrometer.
Using comparative genomic approaches, we have identified a dozen of kinases and phosphatases that are expressed in C. elegans and Chlamydomonas cilia, while their effect in cilium genesis and IFT have not yet been studied. Critically, we identified two kinases PGK-1 and GCK-2 largely affecting cilia growth and IFT, and we were able to link these obervations to changes in post-translational modification of tubulin in these cilia (Shanmugam, M. M., P. Bhan et al., Mol. Cell Biol., 2018):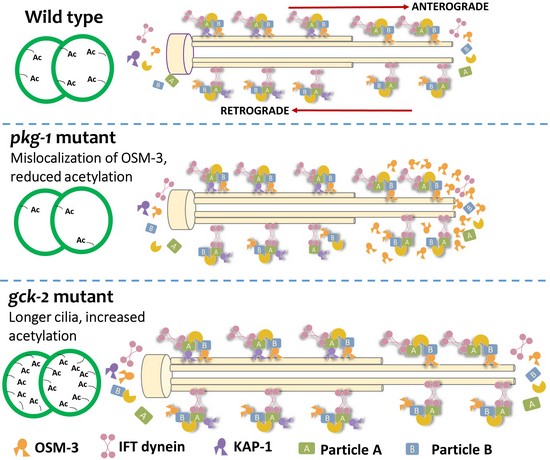
==============================================================================================
Our publications on the regulation of organelle transport have been cited in various high impact review articles:
- Khalil et al., 2024. Nat Rev Neurol 20, 269-287
- Leterrier et al., 2017. Nat Rev Neurosci 18, 713-726
- Sheng et al., 2012. Nat Rev Neurosci 13, 77-93
- MacAskill et al., 2010. Trends Cell Biol 20, 102-112
- Godsel et al., 2008. Trends Cell Biol 18, 28-37
- Boldogh et al., 2007. Trends Cell Biol 17, 502-510
- Toivola et al., 2005. Trends Cell Biol 15, 608-617
- Barnhart et al., 2016. Curr Opin Cell Biol 38, 90-99
- Goldman et al., 2008. Curr Opin Cell Biol 20, 28-34
- Yan et al., 2012. Curr Opin Neurobiol 22, 431-437
- Sigrist et al., 2011. Curr Opin Neurobiol 21, 144-150
- Franze et al., 2011. Curr Opin Genetics Develop 21, 530-537
- De Vos et al., 2008. Annu Rev Neurosci. 31, 151-173
- Götz et al., 2013. Front Neurol 4, 72
- Castellanos et al., 2020. Front Cell Neurosci 14, 594975
- Xie et al., 2021. Front Cell Develop Biol 9, 653381.
- Kotaich et al., 2023. Front Cell Develop Biol 11, 1275155.
- Zha et al., 2021. Front Mol Biosci7, 632122
- Scarinci et al., 2022. Front Physiol 13, 995473.
- Hu et al., 2020, Front Aging Neurosci 12, 605961.