DNA Recognition by Eucaryotic Transcription Factors
The TATA box-binding protein
was first isolated and purified from yeast in 1988 - with a MW of 27 kDa.
The C-terminal domain contains
- all the functions essential for normal yeast cell growth.
- two homologous repeats of 88
amino acids
- binds to TATA consensus sequence with a Kd
in
the
nanomolar range.
Two similar domains form a saddle-shaped
molecule
with stirrups.
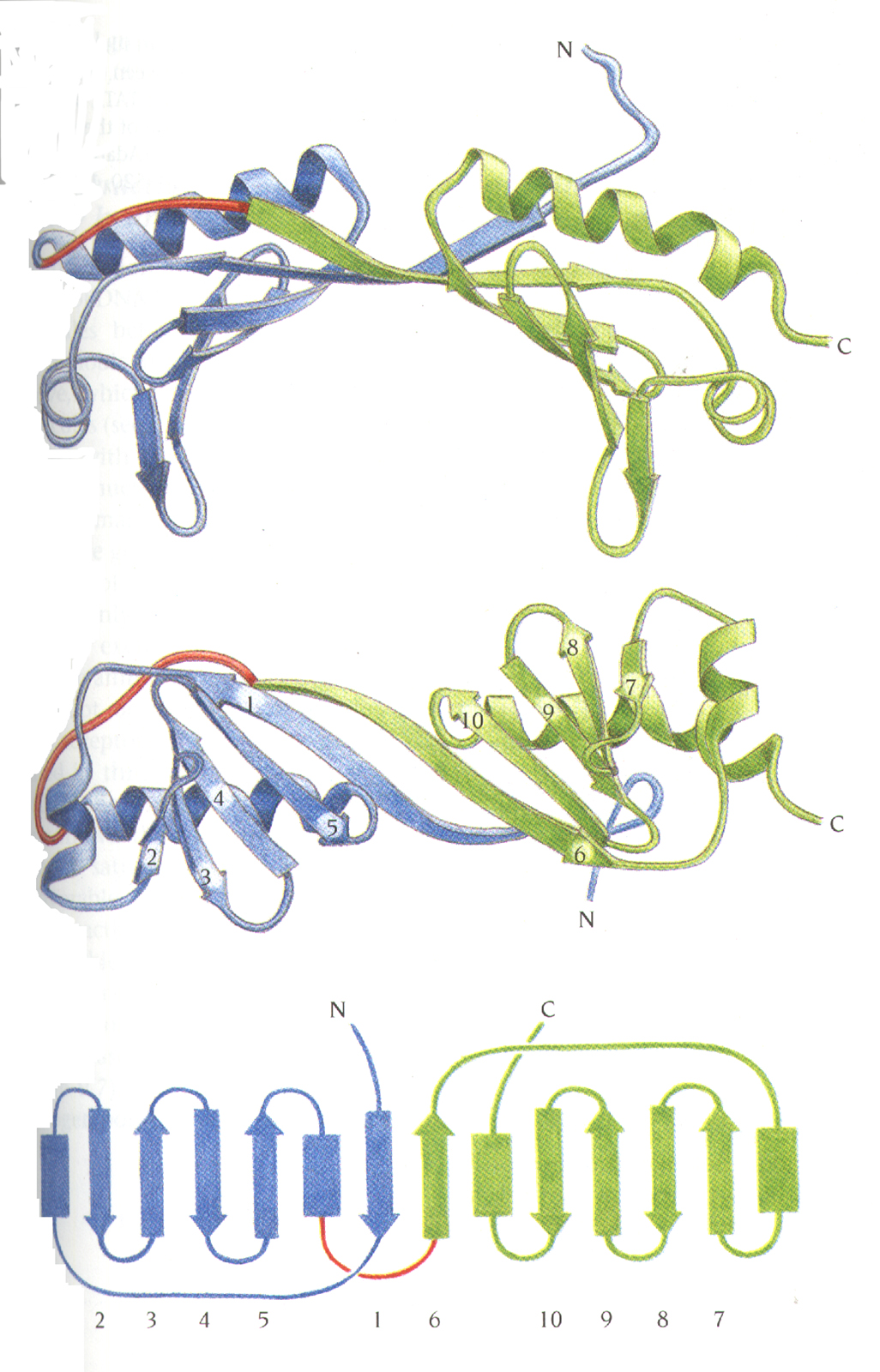
A b sheet
in TBP forms the DNA-binding site
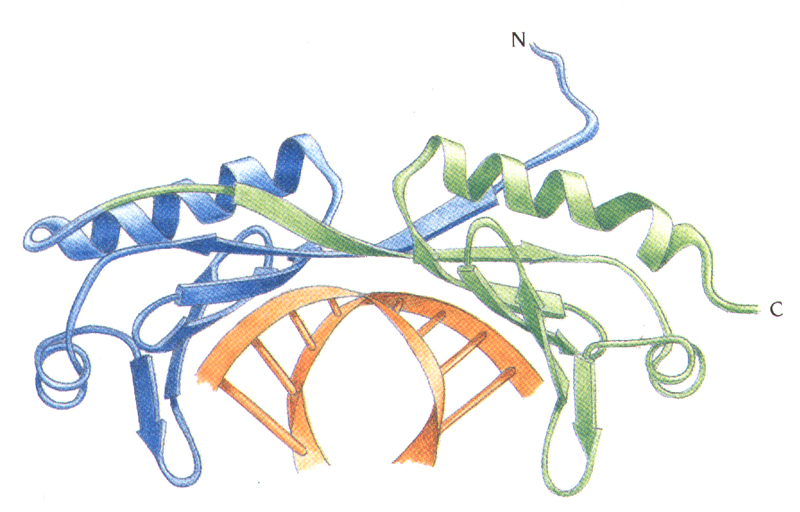
TBP binds in the minor groove and
induces large structural changes in DNA
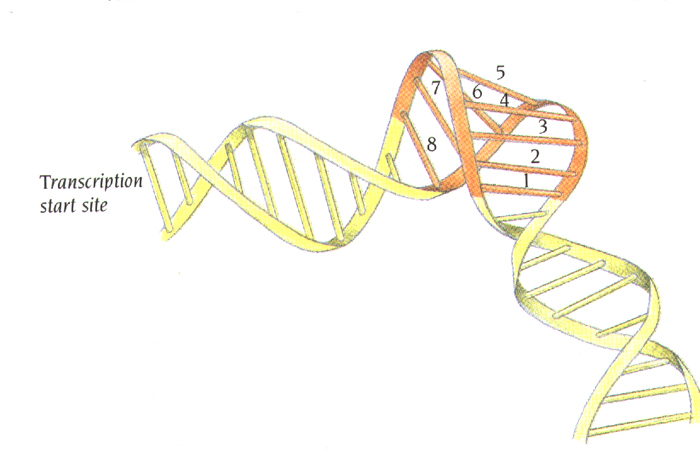
induces DNA bending ~ 100
degrees.
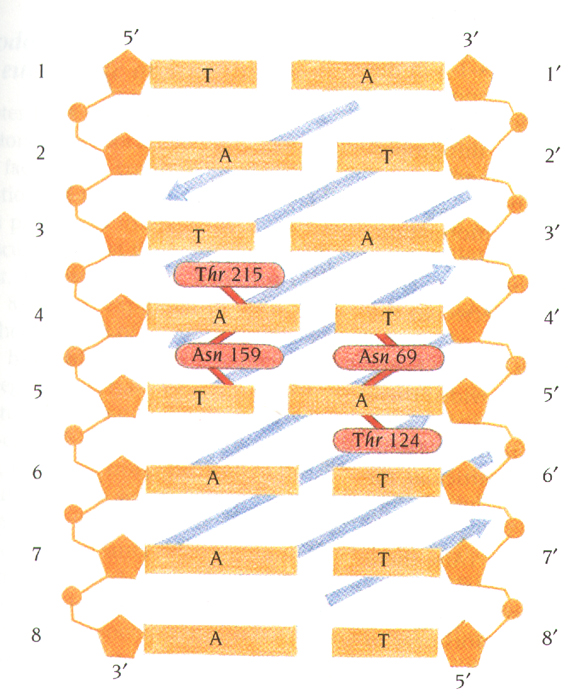
The interaction area between TBP and TATA box is mainly hydrophobic
The only sequence-specific hydrogen bonds between TBP side
chains and the bases in the minor groove occur at the very center of the
TATA box.
Functional implications of the
distortion of DNA by TBP
| Up | Next
|



