|
ggggg |



|
首頁 |
|
研究方向 |
|
成員 |
|
著作 |
|
教學課程 |
|
Publications Chen, Y. S., Lim, A. C., Chen, M. H., Quinlan, R. A. and Perng, M. D. (2011) Alexander disease causing mutations in the C-terminal domain of GFAP are deleterious both to assembly and network formation with the potential to both activate caspase 3 and decrease cell viability. Experimental Cell Research. 317(16): 2252-2266. Saunter, C. D, Perng, M. D., Love, G. D., Quinlan and R. A. (2009) Stochastically determined directed movement explains the dominant small-scale mitochondrial movements within non-neuronal tissue culture cells. FEBS Lett. [Epub ahead of print] Perng, M. D., Wen, S. F., Gibbon, T., Middledrop, J., Sluijs, J., Hol, E. M. and Quinlan, R. A. (2008) Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol. Cell. 19: 4521-4533. Perng, M. D., Zhang, Q. and Quinlan, R. A. (2007) Insights into the beaded filaments of the eye lens. Exp. Cell Res. 313: 2180-2188. Perng, M. D., Su, M., Wen, S. F., Li, R., Gibbon, T., Prescott, A. R., Brenner, M. and Quinlan, R. A. (2006) The Alexander disease-causing GFAP mutant, R416W, accumulates into Rosenthal fibers by a pathway involving filament aggregation and the association of alphaB-crystallin and HSP27. Am. J. Hum. Genet. 79: 197-213. Hsiao, V. C., Tian, R., Long, H., Perng, M. D., Brenner, M., Quinlan, R.A. and Goldman, J. E. (2005) Alexander-disease mutation of GFAP causes filament disorganisation and decreased solubility of GFAP. J. Cell Sci. 118:2057-2065. Perng, M. D. and Quinlan, R. A. (2005) Seeing is believing! The optical properties of the eye lens are dependent upon a functional intermediate filament cytoskeleton. Exp. Cell Res. 305:1-9. Treweek, T. M., Rekas, A., Lindner, R. A., Walker, M. J., Aqualina, J. A., Robinson C. V., Horwitz, J., Perng, M. D., Quinlan, R. A. and Carver, J. A. (2005) The R120G alphaBcrystallin promotes the unfolding of reduced a-lactabumin and is inherently unstable. FEBS J. 272: 711-724. Perng, M. D., Sandilands, A., Kuszak, J., Dahm, R., Wegener, A., Prescott A. R. and Quinlan, R. A. (2004) The intermediate filament system in the eye lens. Methods Cell Biol. 78: 597-624. Golenhofen, N., Perng, M. D., Quinlan, R. A. and Drenckhahn, D. (2004)Comparison of the small heat shock proteins alpha B-crystallin, MKBP, HSP25, HSP20 and cvHSP in heart and skeletal muscle. Histochem. Cell Biol. 122:415-425. Perng, M. D. and R. A. Quinlan (2004) Neuronal diseases: Small heat shock proteins calm your nerves. Curr. Biol. 14: R625-R626. Perng, M. D., Wen, S. F., van den Ijssel, P., Prescott A. R. and Quinlan, R. A. (2004) Desmin aggregate formation by R120G aB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol. Biol. Cell 15:2335-2346. |

|
在這裡放置您的商務標語。 |
|
公司名稱 |
|
機構 |


|
研究方向:
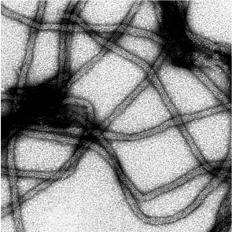
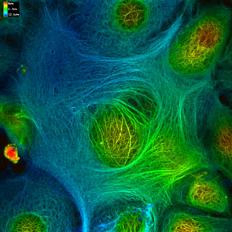
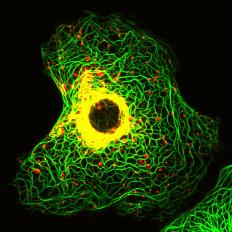
1.神經膠質纖維酸性蛋白質GFAP基因突變與逆境反應在亞歷山大氏症所扮演的角色 2.GFAP基因突變對於中間型蛋白絲的聚合及形成絲狀纖維能力的影響 3.粒腺體與肌間絲蛋白之交互作用在肌病變中所扮演的角色
最新著作:
Chen, Y. S., Lim, A. C., Chen, M. H., Quinlan, R. A. and Perng, M. D. (2011) Alexander disease causing mutations in the C-terminal domain of GFAP are deleterious both to assembly and network formation with the potential to both activate caspase 3 and decrease cell viability. Experimental Cell Research. 317(16): 2252-2266. Saunter, C. D, Perng, M. D., Love, G. D., Quinlan and R. A. (2009) Stochastically determined directed movement explains the dominant small-scale mitochondrial movements within non-neuronal tissue culture cells. FEBS Lett. [Epub ahead of print] Perng, M. D., Wen, S. F., Gibbon, T., Middledrop, J., Sluijs, J., Hol, E. M. and Quinlan, R. A. (2008) Glial fibrillary acidic protein filaments can tolerate the incorporation of assembly-compromised GFAP-delta, but with consequences for filament organization and alphaB-crystallin association. Mol Biol. Cell. 19: 4521-4533. Perng, M. D., Zhang, Q. and Quinlan, R. A. (2007) Insights into the beaded filaments of the eye lens. Exp. Cell Res. 313: 2180-2188. |
|
分子醫學研究所 彭明德老師 實驗室 |