An exact theory of electrophoresis seems almost impossible to develop. Serious difficulties arise because electrophoresis must almost always be carried out in aqueous solutions containing buffer ions and salts in addition to the macromolecule of interest; at the very least the counterions that have dissociated from the macromolecule to provide its charge must be present. To simplify the problem, we may write the basic equation for electrophoresis in exactly the same fashion as for sedimentation; the only difference is the source of force. The force experienced by a particle in an electrical field is given by Coulomb's law,
F = ZeE
where Z is the number of charge, e is the magnitude of the electronic charge, and E is the electrical field in units of potential per centimeter. Since the resistance to motion is given by -fv, where f is the frictional factor, and v the velocity, the condition that the net force on the particle be zero in steady in steady motion tells us:
fv = ZeE
The equation above can be rearranged to define an electrophoresis mobility U, a quantity very analogous to the sedimentation coefficient.
U = v/E = Ze/f
As with the sedimentation coefficient, U is the ratio of velocity to the strength of the driving field. If the particle happens to be spherical, Stroke's law applies and we can write
U = Ze/6phR
where R is the particle radius and h the solvent viscosity.
| Some media for zonal electrophoresis | ||
| Medium | Conditions | Principle Uses |
| Paper | Filter paper moistened with buffer, placed between electrodes | Small molecules: amino acids or nucleotides |
| Polyacrylamide gel | Cast in tubes or slabs; cross-linked | Proteins and nucleics |
| Agarose gel | As polyacrylamide, but no cross-linking | Vary large proteins, nucleic acids, nucleoproteins. etc. |
A macromolecule cannot move as easily through a network as ia can when free in solution. In fact, a very simple relationship has been discovered between relative mobility and gel concentration. Such graphs are called Ferguson plots:
log Uri = log U0ri - kiC
where C is the gel concentration and U0ri. is the relative mobility of component i whrn C = 0. For a given gel concentration, there will usually be some molecular weight range in whiich log Mi and Uri are approximately linearly related. One typical type of molecule that could approximate this kind of behavior is a long rod, which charge proportional to its length, e.g., DNA. The frictional coefficient of a thin rod of length L is given by
f ~ 3phL/ln(L/b)
If the charge z is proportional to the length L, we get,
U ~ KL/f = (K/3ph)ln(L/b)
At large value of L/b, U will change very slowly with increasing L.
[Example]
Resolution can be greatly improved using isoelectric focusing. In this technique the support gel maintains a pH gradient. As a protein migrates down the gel, it reaches a pH that is equal to its isoelectric point. At this pH the protein is neutral and no longer migrates, i.e., it is focused into a sharp band on the gel.
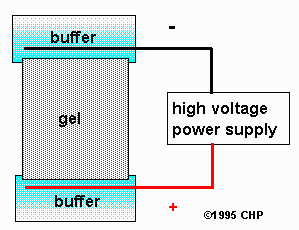
Schematic of zone electrophoresis apparatus
