f0 = 6phR
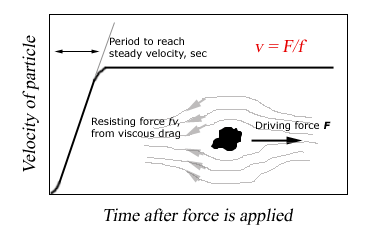
A schematic picture of the behavior of a particle suddenly subjected to a force F.
There are many powerful techniques, such as circular dichroism (CD), fluorescence, X-ray diffraction and NMR spectroscopy, that can help us investigate the chemical and physical properties of biomolecules. However, before we can apply these techniques to those biological systems of interest, we must first purify these macromolecules from the multitude of molecules, both large and small, found in most biochemical preparations. There are several questions we need to answer:
In summary, the purification of biomolecules often deals with several factors:
If we consider the separation as a mechanical problem, in solution, then a solute molecule can be separated by applying an external force F, mechanically or electrically, onto it. If the force is large enough, the external acceleration will overcome the thermo-Brownian motion and moving toward one direction. Yet, the acceleration will last for only an exceedingly short period of time, for as its velocity increase, the molecule will experience an increasing frictional resistance to surrounding medium, i.e., frictional force -fv, where v is the velocity, and f is the constant frictional coefficient of the molecule, which is determined by the physical properties listed above.
An equilibrium state will reach to a constant velocity v when the two forces are equal:
-fv + F = 0, or F = fv
Thus, we can determine the frictional coefficient by measuring the external force and steady velocity.
The frictional resistance arises because of the viscosity of the fluid. However, the frictional coefficient is difficult to calculate, and exact expression have been obtained only for few simple particle shapes. For example, for a sphere of radius R
f0 = 6phR
A schematic picture of the behavior of a particle suddenly subjected to a force F.
[Next]