A = a(![]() ) * b * c
) * b * c
The Beer-Lambert law (or Beer's law) is the linear relationship between absorbance and concentration of an absorbing species. The general Beer-Lambert law is usually written as:
A = a(
) * b * c
where A is the measured absorbance, a(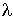 ) is a wavelength-dependent
absorptivity coefficient, b is the path
length, and c is the analyte concentration.
When working in concentration units of molarity, the Beer-Lambert law
is written as:
) is a wavelength-dependent
absorptivity coefficient, b is the path
length, and c is the analyte concentration.
When working in concentration units of molarity, the Beer-Lambert law
is written as:
A =
* b * c
where 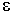 is the wavelength-dependent molar
absorptivity coefficient with units of M-1 cm-1. Data
are frequently reported in percent transmission (I/I0 * 100) or in
absorbannce [A = log (I/I0)]. The latter is particularly convenient.
[common coefficients of near-ultraviolet absorption
bands of some amino acids and nucleotides]
is the wavelength-dependent molar
absorptivity coefficient with units of M-1 cm-1. Data
are frequently reported in percent transmission (I/I0 * 100) or in
absorbannce [A = log (I/I0)]. The latter is particularly convenient.
[common coefficients of near-ultraviolet absorption
bands of some amino acids and nucleotides]
Sometimes the extinction coefficient is given in other units; for example,
A = E1% * b * c
where the concentration C is in gram per 100 ml of solution. This useful when the molecular weight of the solute is unknown or uncertain.
Q: Cytosine has a molar extinction coefficient of 6*103 at 270 nm at pH 7. Calculate the absorbance and percent transmission of 1*10-4 and 1*10-3 M cytosine solution in a 1-mm cell. [Solution....]
Q: A protein with extinction coefficient E1% = 16 yields an absorbance of 0.73 when measured in a 0.5-cm cell. Calculate the weight concentration. [Solution....]
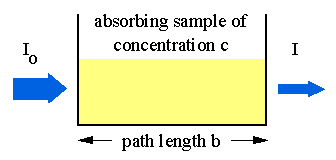
Experimental measurements are usually made in terms of transmittance (T), which is defined as:
T = I / Io
where I is the light intensity after it passes through the sample and Io is the initial light intensity. The relation between A and T is:
A = -log T = - log (I / Io).
Absorption of light by a sample
Modern absorption instruments can usually display the data as either transmittance,
%-transmittance, or absorbance. An unknown concentration of an analyte can be
determined by measuring the amount of light that a sample absorbs and applying
Beer's law. If the absorptivity coefficient is not known, the unknown concentration
can be determined using a working curve of absorbance versus concentration derived
from standards.
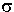 , represents the
effective area seen by a photon of frequency w. If the frequency
of the light is far from resonance, the area is approximately 0, and if
w is close to resonance the area is a maximum. Taking an infinitesimal
slab, dz, of sample:
, represents the
effective area seen by a photon of frequency w. If the frequency
of the light is far from resonance, the area is approximately 0, and if
w is close to resonance the area is a maximum. Taking an infinitesimal
slab, dz, of sample:
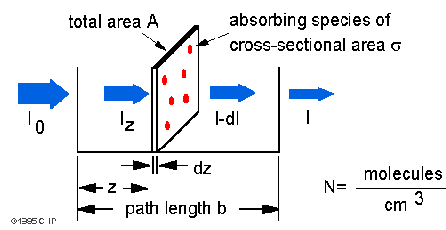
Io is the intensity entering the sample at z=0, Iz
is the intensity entering the infinitesimal slab at z, dI is the intensity
absorbed in the slab, and I is the intensity of light leaving the sample.
Then, the total opaque area on the slab due to the absorbers is 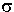 * N * A * dz. Then, the fraction of photons absorbed will be
* N * A * dz. Then, the fraction of photons absorbed will be 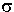 * N * A * dz / A so,
* N * A * dz / A so,
dI / Iz = -
* N * dz
Integrating this equation from z = 0 to z = b gives:
ln(I) - ln(Io) = -
* N * b
or - ln(I / Io) =
* N * b.
Since N (molecules/cm3) * (1 mole / 6.023x1023 molecules) * 1000 cm3 / liter = c (moles/liter) and 2.303 * log(x) = ln(x) then
- log(I / Io) =
* (6.023x1020 / 2.303) * c * b
- log(I / Io) = A =
* b * c
where 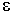 =
= 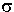 *
(6.023x1020 / 2.303) =
*
(6.023x1020 / 2.303) = 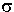 * 2.61x1020
* 2.61x1020
Typical cross-sections and molar absorptivities are:
(cm2)
(M-1 cm-1) absorption - atoms 10-12 3x108 molecules 10-16 3x104 infrared 10-19 3x10 Raman scattering 10-29 3x10-9
[BACK]