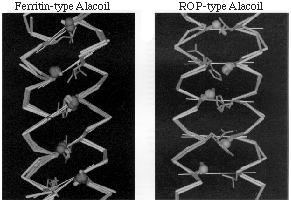

| A: Four long examples of the ferritin-type Alacoil structure, superimposed in side view. The Calpha backbones are shown in gold, plus the small F side chains (Ala or Ser, in green, with a blue ball at the Cbeta position) for the critical "a" heptad position that packs tightest against the opposite helix, and gray side chains for the "d" position. Thin white lines join the "a" Calpha to a point halfway between "d" and "e," to show the diagonal nature of the closest side-chain contacts. Note that the helix turns appear to interdigitate: the vertical offset between equivalent "a" positions on the two helices is 0.25 times the heptad repeat, or half of a helix turn. B: The only two long examples of the ROP-type Alacoil structure superimposed in side view, showing Calpha backbones in gold, the small side chains in the critical "d" heptad position of tightest packing in orange with green balls at the Cbeta atoms, and the "a" side chains opposite them in gray. Thin white lines join the "d" Calpha to a point midway between "g" and "a." Contacting turns lie directly opposite each other on the two heli
|