|
Helicobacter pylori is a microaerophilic gram-negative bacterium that
colonizes the stomachs of an estimated half of all humans. Four diseases are now widely
acknowledged to be caused by H. pylori: duodenal ulcer, gastric
ulcer, adenocarcinoma of the distal stomach, and gastric mucosa-associated
lymphoid tissue lymphoma. Helicobacter
pylori neutrophil-activating protein (HP-NAP), a virulence factor of
H. pylori, plays an important role in pathogenesis of H. pylori
infection. HP-NAP was first found to be able to stimulate the production of
reactive oxygen species (ROS) in neutrophils and promote adhesion of
neutrophils to endothelial cells.
Now, HP-NAP is known to play a role not only in innate immunity but also
in adaptive immunity. HP-NAP has been shown as a ligand binding to
Toll-like receptor 2 (TLR2) and an unidentified G protein-coupled receptor
(GPCR). The engagement of TLR2
and GPCR seems to be related to HP-NAP-induced production of ROS and
cytokines by leukocytes, respectively (Fig.4). However, the molecular mechanisms by
which HP-NAP activates these two receptors are not clear. Molecular docking technique has been
used to predict the binding site between HP-NAP and TLR2. Several possible docking
conformations are shown in Fig. 5.
The possible amino acid residues involved in the interaction of
HP-NAP and TLR2 will be subjected to site-directed mutagenesis. The mutated HP-NAP will be expressed
in the Escherichia coli expression system established in our lab
(Wang et al., 2008, Biochem Biophys Res Commun. 377:
52-56). The identities of the
amino acid residues responsible for the interaction of HP-NAP and TLR2
receptor will be further analyzed by examining the ability of the HP-NAP
mutants to bind and to activate the receptor. Whether these amino acid residues
are also involved in HP-NAP-induced GPCR activation will also be determined
by examining if those HP-NAP mutants could stimulate ROS production in
human neutrophils.

Fig. 4. The cellular responses of HP-NAP acting on its
receptors. HP-NAP could induce leukocytes, such as neutrophils and monocytes, to produce cytokines
through TLR2 activation or to release ROS through GPCR signaling pathway.
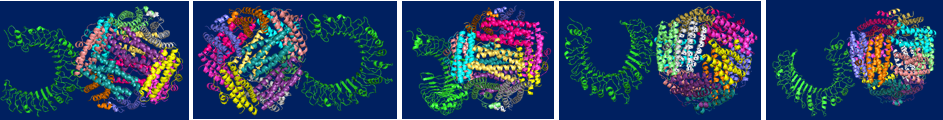
Fig.
5. The possible docking conformation of TLR2 paired with HP-NAP. The TLR2/HP-NAP
docking complexes are sorted into five groups according to its different
orientation and binding site on the complex. Molecular docking is predicted
by docking servers of Patchdock and Gramm-X.
Back
|