Electromagnetic Radiation
Introduction
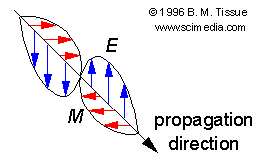
Electromagnetic radiation is a transverse energy wave that is composed of
an oscillating electric field component, E, and an oscillating magnetic
field component, M. The electric and magnetic fields are orthogonal
to each other, and they are orthogonal to the direction of propogation of
the wave. A wave is described by the wavelength, 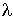 ,
which is the physical length of one complete oscillation, and the frequency, ,
which is the physical length of one complete oscillation, and the frequency, 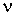 ,
which is the number of oscillations per second. The figure shows one wavelength
of a wave of light. The names we give electromagnetic radiation for different
wavelength and frequency ranges are listed in the electromagnetic
spectrum document. ,
which is the number of oscillations per second. The figure shows one wavelength
of a wave of light. The names we give electromagnetic radiation for different
wavelength and frequency ranges are listed in the electromagnetic
spectrum document. |
Schematic of an electromagnetic wave
 |
Velocity of light
Electromagnetic waves travel through a vacuum at a constant velocity of
2.99792x108 m/s, which is known as the speed of light, c. The
relationship between the speed of light, wavelength, and frequency is:
c = 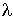
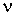
When light passes through other media, the velocity of light decreases.
For a given frequency of light, the wavelength also must decrease. This
decrease in velocity is quantitated by the refractive index, n, which is
the ratio of c to the velocity of light in another medium, v:
n = c / v
| Since the velocity of light is lower in other media than in a vacuum,
n is always a number greater than one. The table lists the refractive index
of several examples. Refractive index is an intrinsic physical property
of a substance, and can be used to monitor purity or the concentration
of a solute in a solution. The refractive index of a material is measured
with a refractometer, and is usually made versus air. If the precision
warrants, the measurements can be corrected for vacuum. Note that the difference
between nair and nvacuum is only significant in the
fourth decimal place.
For anisotropic materials, such as quartz crystals, light of different
polarizations (see below) will experience different refractive indices.
These indices are called the ordinary refractive index, no,
and the extraordinary refractive index, ne. |
| medium |
n* |
| air |
1.0003 |
| water |
1.333 |
| 50% sucrose in water |
1.420 |
| carbon disulfide |
1.628 |
| crystalline quartz |
1.544 (no)
1.553 (ne) |
| diamond |
2.417 |
*measured with 589.3 nm light |
Polarization
An incoherent light source, such as the hot filament of a light bulb, consists
of multiple, randomly oriented light emitters, which produce electromagnetic
waves with their electric-field vector oriented in all directions. The
resulting light emission is called unpolarized light.
Linearly (or plane) polarized light is light in which the electric-field vector
is oscillating in only one direction. Linearly polarized light is produced by
isolating one orientation of the electric field with a polarizer,
or from lasers that contain polarized optical components.
Circularly polarized light is light in which the electric field vector
is rotating around the axis of light propogation. The electric field vector
can rotate in either the right or left direction (as viewed in the direction
of light propogation), and the light is called right circularly polarized
or left circularly polarized, respectively.
Wave-particle duality
Electromagnetic radiation shows both wave and particle characteristics
depending on how the radiation is observed. Einstein first postulated that
the energy of radiation is quantized and that radiation is composed of
energy packets that were later named photons. The energy, E, of one photon
depends on its frequency (or wavelength):
E = h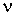 = hc /
= hc /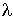
where h is Planck's constant (6.62618x10-34 Js), 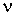 is the frequency of the radiation, c is the speed of light, and
is the frequency of the radiation, c is the speed of light, and 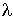 is wavelength.
is wavelength.
de Broglie equation
Moving particles; such as electrons, protons, and neutrons; have wave properties
as described by the de Broglie equation:
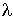 = h / p
= h / p
where 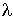 is wavelength, h is Planck's constant,
and p is the momentum of the particle. Beams of particles can therefore
show wave effects such as interference.
is wavelength, h is Planck's constant,
and p is the momentum of the particle. Beams of particles can therefore
show wave effects such as interference.
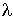 ,
which is the physical length of one complete oscillation, and the frequency,
,
which is the physical length of one complete oscillation, and the frequency, 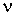 ,
which is the number of oscillations per second. The figure shows one wavelength
of a wave of light. The names we give electromagnetic radiation for different
wavelength and frequency ranges are listed in the electromagnetic
spectrum document.
,
which is the number of oscillations per second. The figure shows one wavelength
of a wave of light. The names we give electromagnetic radiation for different
wavelength and frequency ranges are listed in the electromagnetic
spectrum document.