Introduction to Mass Spectrometry (MS)
Introduction
Mass spectrometers use the difference in mass-to-charge ratio (m/e) of
ionized atoms or molecules to separate them from each other. Mass spectrometry
is therefore useful for quantitation of atoms or molecules and also for determining
chemical and structural information about molecules. Molecules have distinctive
fragmentation patterns that provide structural information to identify structural
components.
The general operation of a mass spectrometer is:
- create gas-phase ions
- separate the ions in space or time based on their
mass-to-charge ratio
- measure the quantity of ions of each mass-to-charge
ratio [How to derive m/e ratio?]
The ion separation power of a mass spectrometer is described by the resolution,
which is defined as:
R = m / 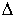 m,
m,
where m is the ion mass and 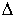 m
is the difference in mass between two resolvable peaks in a mass spectrum. E.g.,
a mass spectrometer with a resolution of 1000 can resolve an ion with a m/e
of 100.0 from an ion with an m/e of 100.1.
m
is the difference in mass between two resolvable peaks in a mass spectrum. E.g.,
a mass spectrometer with a resolution of 1000 can resolve an ion with a m/e
of 100.0 from an ion with an m/e of 100.1.
Instrumentation
In general a mass spectrometer consists of an ion source, a mass-selective analyzer,
and an ion detector. Since mass spectrometers create and manipulate gas-phase
ions, they operate in a high-vacuum system. The magnetic-sector,
quadrupole, and time-of-flight designs also require extraction and acceleration
ion optics to transfer ions from the source region into the mass analyzer. The
details of mass analyzer designs are discussed in the individual documents listed
below. Basic descriptions of sample introduction/ionization and ion detection
are discussed in separate documents on ionization methods
and ion detectors, respectively.
[BACK]
![]() m,
m,m,
![]() m
is the difference in mass between two resolvable peaks in a mass spectrum. E.g.,
a mass spectrometer with a resolution of 1000 can resolve an ion with a m/e
of 100.0 from an ion with an m/e of 100.1.
m
is the difference in mass between two resolvable peaks in a mass spectrum. E.g.,
a mass spectrometer with a resolution of 1000 can resolve an ion with a m/e
of 100.0 from an ion with an m/e of 100.1.