X-ray Crystallography is the principal method by which the detailed 3-dimensional structures of molecules - especially the molecules of living systems - have been discovered.
Why X-rays? X-rays and visible light are both electromagnetic radiation
and have a wave-like nature. But the wavelength of visible light is more than
a thousand times longer than the distances within molecules such as the length
of the bonds that join atoms together or the separation of the bases in a nucleic
acid chain. To get information about this kind of detail, we have to 'probe'
the molecules with radiation whose wavelength is of a similar order to the dimensions
involved and X-rays are just right for this purpose - they have wavelengths
of about 1-2 Angstrom units (0.1 - 0.2 x 10-9 m). X-rays falling on a molecule
are scattered by the atoms in it (strictly speaking by the atoms' electrons).
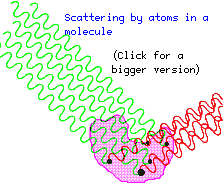 The diagram
shows a beam of X-rays falling on a molecule (such as a protein molecule) and
the rays that have been scattered in a particular direction by some of the atoms
in the molecule. You can see that the incoming beam is made up from rays that
are all in step (in phase) with each other, but the scattered rays are no longer
in step, having travelled different distances. The result is that the rays tend
to cancel each other out, as the peaks of one ray fall near the troughs of another,
and so on. The strength of the scattered beam is therefore sensitive to the
spatial arrangement of the atoms in the molecule.
The diagram
shows a beam of X-rays falling on a molecule (such as a protein molecule) and
the rays that have been scattered in a particular direction by some of the atoms
in the molecule. You can see that the incoming beam is made up from rays that
are all in step (in phase) with each other, but the scattered rays are no longer
in step, having travelled different distances. The result is that the rays tend
to cancel each other out, as the peaks of one ray fall near the troughs of another,
and so on. The strength of the scattered beam is therefore sensitive to the
spatial arrangement of the atoms in the molecule.
Why crystals? A single molecule is very small and would scatter the
X-rays very weakly. In a crystal, molecules are lined up in a regular way, like
soldiers in a platoon, and their scattering of X-rays adds up like the sound
of the footsteps of the marching soldiers.
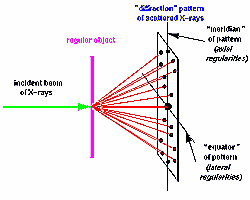 The molecules in some important biological materials
are lined up naturally. These include hair, wool and silk, fibrous protein
molecules that, because of their inner regularity and resultant strength
were used for textiles before artificial materials such as nylon and terylene
became available - even now they remain widely used, often in combination with
the newer substances. The consequence of their internal regularity is that they
form fibres and, when a beam of X-rays falls upon a fibre, it is scattered strongly
only in those directions in which the contributions from different parts of
the molecules add up. The scattering pattern can be recorded on a piece of photographic
film placed behind the specimen, and the positions of the strong spots can then
be measured, giving information about the regularities within the fibre. Astbury,
one of the British pioneers of Biophysics working in Leeds, found that the pattern
given by hair or wool changed when the fibre was stretched, showing that the
molecules themselves had been pulled out from a tightly-wound, compact, form
into an elongated shape.
The molecules in some important biological materials
are lined up naturally. These include hair, wool and silk, fibrous protein
molecules that, because of their inner regularity and resultant strength
were used for textiles before artificial materials such as nylon and terylene
became available - even now they remain widely used, often in combination with
the newer substances. The consequence of their internal regularity is that they
form fibres and, when a beam of X-rays falls upon a fibre, it is scattered strongly
only in those directions in which the contributions from different parts of
the molecules add up. The scattering pattern can be recorded on a piece of photographic
film placed behind the specimen, and the positions of the strong spots can then
be measured, giving information about the regularities within the fibre. Astbury,
one of the British pioneers of Biophysics working in Leeds, found that the pattern
given by hair or wool changed when the fibre was stretched, showing that the
molecules themselves had been pulled out from a tightly-wound, compact, form
into an elongated shape.
 DNA
is a very long molecule and does not naturally form long, thin fibres. But it
is possible to extract DNA from cells in the form of a viscous gel; if a needle
is dipped into the gel and slowly wound up, it drags out a DNA fibre in which
many molecules are lined up parallel to each other. The X-ray patterns given
by DNA fibres show a pair of strong arcs along their vertical axis; Astbury
realised that their position indicated a very regular periodicity of 3.4 along
the axis of the fibre and that this figure was similar to the thickness of the
DNA bases; he therefore suggested that the bases were stacked on top of each
other "like a pile of pennies". He was quite right, but the well-known double
helix structure had to await much better X-ray pictures (obtained by Wilkins,
Franklin and colleagues at King's College, London) and the realisation by Crick
and Watson (in Cambridge) that the bases were in pairs, joining two backbones
running in opposite directions.
DNA
is a very long molecule and does not naturally form long, thin fibres. But it
is possible to extract DNA from cells in the form of a viscous gel; if a needle
is dipped into the gel and slowly wound up, it drags out a DNA fibre in which
many molecules are lined up parallel to each other. The X-ray patterns given
by DNA fibres show a pair of strong arcs along their vertical axis; Astbury
realised that their position indicated a very regular periodicity of 3.4 along
the axis of the fibre and that this figure was similar to the thickness of the
DNA bases; he therefore suggested that the bases were stacked on top of each
other "like a pile of pennies". He was quite right, but the well-known double
helix structure had to await much better X-ray pictures (obtained by Wilkins,
Franklin and colleagues at King's College, London) and the realisation by Crick
and Watson (in Cambridge) that the bases were in pairs, joining two backbones
running in opposite directions.
 Globular
proteins (such as enzymes, hormones and antibodies), unlike the elongated
protein molecules that nature has designed to form fibres, are folded up into
compact shapes. Fortunately, it is often possible to form crystals of them,
in which the molecules are regularly arranged in a 3-dimensional lattice with
water filling the spaces between the protein molecules.
Globular
proteins (such as enzymes, hormones and antibodies), unlike the elongated
protein molecules that nature has designed to form fibres, are folded up into
compact shapes. Fortunately, it is often possible to form crystals of them,
in which the molecules are regularly arranged in a 3-dimensional lattice with
water filling the spaces between the protein molecules.
X-rays falling on such crystals are scattered in a very regular way; the regular
spacings between the spots just tell us about the distances between the molecules
in the crystal lattice, but the variations in strength of the spots give information
about the arrangement of the atoms within each molecule. Unfortunately, this
is only half of the information required to determine the atom positions completely,
because each X-ray beam falling on the film has two properties - its amplitude
(which we can measure) and its phase relative to all the other beams (which
we cannot). Various cunning methods have been found to overcome this problem,
one being to modify the protein molecules in the crystal at one or two positions
by adding marker atoms which scatter X-rays strongly.
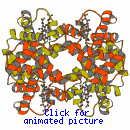 Kendrew (working in
Cambridge) and his colleagues were the first to elucidate the 3- dimensional
structure of a globular protein, that of myoglobin, the protein that
stores oxygen in muscle tissues. Shortly afterwards, Perutz and his team (also
in Cambridge) found the structure of haemoglobin, which carries oxygen
in the erythrocytes in the blood. Each haemoglobin molecule comprises two pairs
of protein chains, each kind of chain being very similar to a myoglobin chain.
This was the first evidence from 3-dimensional structures of the way in which
complicated molecules have evolved from simpler ones, so giving rise to novel
properties; in the case of haemoglobin, it is vitally important that the protein
is capable of picking up oxygen molecules in the lungs and releasing them in
the muscle tissues, where they are required as fuel, and its ability to do this
is a result of a subtle re-arrangement of its four chains in response to small
changes in oxygen pressure and pH (acidity).
Kendrew (working in
Cambridge) and his colleagues were the first to elucidate the 3- dimensional
structure of a globular protein, that of myoglobin, the protein that
stores oxygen in muscle tissues. Shortly afterwards, Perutz and his team (also
in Cambridge) found the structure of haemoglobin, which carries oxygen
in the erythrocytes in the blood. Each haemoglobin molecule comprises two pairs
of protein chains, each kind of chain being very similar to a myoglobin chain.
This was the first evidence from 3-dimensional structures of the way in which
complicated molecules have evolved from simpler ones, so giving rise to novel
properties; in the case of haemoglobin, it is vitally important that the protein
is capable of picking up oxygen molecules in the lungs and releasing them in
the muscle tissues, where they are required as fuel, and its ability to do this
is a result of a subtle re-arrangement of its four chains in response to small
changes in oxygen pressure and pH (acidity).
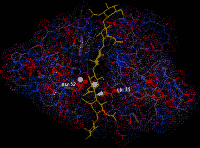 The third
protein of known 3-dimensional structure was an enzyme, lysozyme, studied
by Phillips, Blake, Johnson, North and their colleagues in London. Lysozyme
is a protein with anti-bacterial properties (found in tears and other fluids)
and in this case the studies of the enzyme allowed the mechanism of its action
to be worked out - the enzyme grips its substrate molecule like a pair of pliers
so that active chemical groups on the protein are just in the right place to
attack the substrate.
The third
protein of known 3-dimensional structure was an enzyme, lysozyme, studied
by Phillips, Blake, Johnson, North and their colleagues in London. Lysozyme
is a protein with anti-bacterial properties (found in tears and other fluids)
and in this case the studies of the enzyme allowed the mechanism of its action
to be worked out - the enzyme grips its substrate molecule like a pair of pliers
so that active chemical groups on the protein are just in the right place to
attack the substrate.
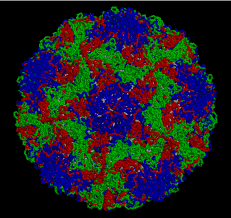 This pioneering research
on biological molecules was almost all carried out in Britain, but activity
is now international and many hundreds of structures are known, including those
of substances of great complexity such as the proteins that control the activity
of DNA and even viruses such as the common cold virus shown here.
This pioneering research
on biological molecules was almost all carried out in Britain, but activity
is now international and many hundreds of structures are known, including those
of substances of great complexity such as the proteins that control the activity
of DNA and even viruses such as the common cold virus shown here.
Although the essentials of the diffraction experiment remain the same, there have been continual developments in instrumentation over the years, such as the availablility of intense X-rays from synchrotrons, which mean that in the future even more rapid and detailed structural studies will be achieved.