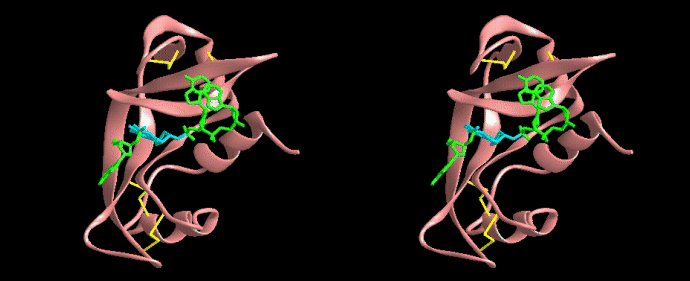Green Fluoresent Protein ( GFP )
*Introduction
*Structure
*Function
*Advantage and disadvantage of GFP
*Introduction:
The green fluoresent protein( GFP ) was made by the jellyfish Aequorea victoria. Jellyfish are generally translucent, but frequently decorated by brightly luminescent structures. In 1991 Prasher et al. Cloned the gene of GFP and the crystal structure of GFP was solved in 1996.
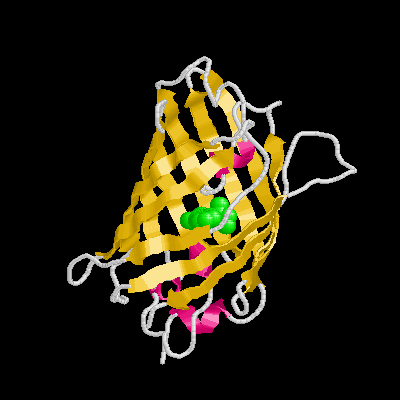
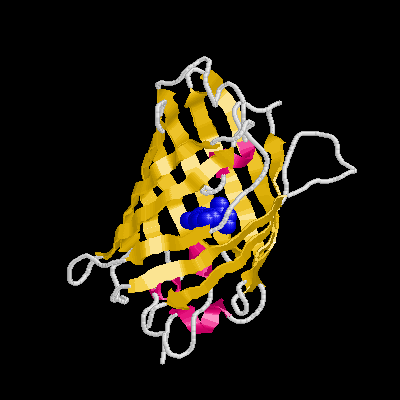
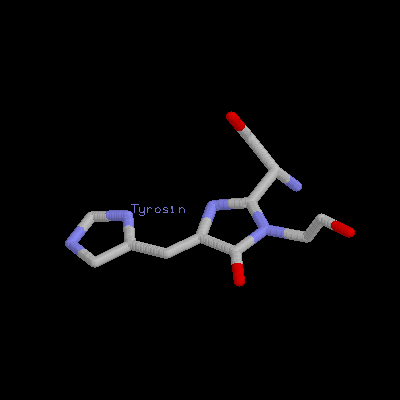
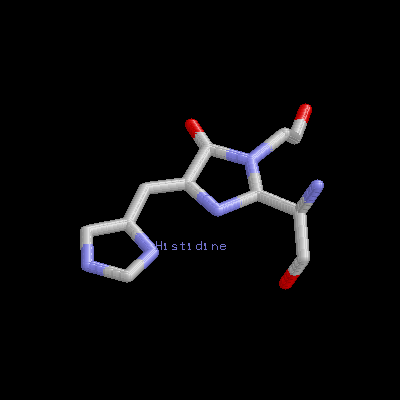
It is a remarkable barrel-like arrangement: eleven b-sheets surround a central a-helix, containing the fluorescent center, which is formed by a hexapeptide. The chromophore itself is formed by a Ser65-Tyr66-Gly67 tripeptide.
GFP is unique among light-emitting proteins in that it dose not require the presence of any cofactors or substrates for the generation of its green light. In the jellyfish, GFP is activated in a calcium-dependent manner when Ca+2 binds another bioluminescent protein, aequorin, which transfers energy indirectly to GFP to trigger the release of green light. The energy transfer can be mimicked experimentally by simple exposure of GFP to standard long-wave ultraviolet light.
*Structure:

*Function:
There are three major contributions in biological science field by using GFP.
Protein tagging
GFP was first used to look into living cells by fluorescence microscopy to monitor protein localization and to visualize dynamic cellular events. A fusion between any cloned gene of interest and GFP can be produced by subcloning techinques and maybe introduced into the organism of interest by transient or stable expression.
Monitoring of gene expression
It allows the characterization of gene expression events at the single cell level. Robinett use the tight and specific binding properties between lac operator and lac repressor to detect the incorporated site in living cells by lac repressor-GFP fusion protein.

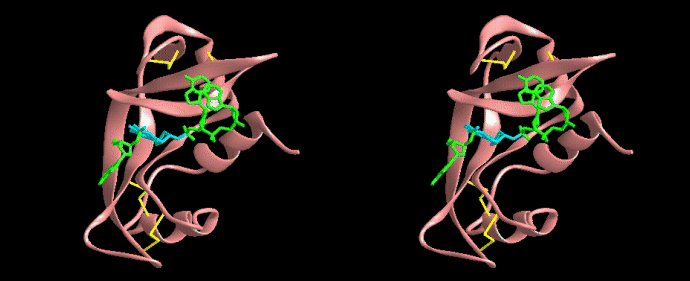
Biological screens
Because the GFP signal can be detected easily and rapidly without manipulation of the sample, perhaps the most promising emerging use of GFP is in selection procedures and in drug and genetic screens. Screening of living cells is especially important in the selection of embryonic stem cells and the production of transgenic animals.
Injection of GFP-labeled tumor-forming cells into nude mice not only labels the tumor, but also allows detection of micrometatastases in locations distant from the primary tumor. This mouse model can now be used both for the study of tumor progression and as an assay system in the search for compounds interfering with metastasis.
GFP will also be useful in drug screens. An impressive example is the establishment of cell lines carry GFP under the control of the HIV-1 long-terminal repeat. Infection of these normally non-fluorescent cells results in the appearance of the GFP signal in the cell due to the transcriptional activation of the HIV-GFP reporter.
*Advantage and disadvantage of GFP :
Advantage of GFP:
Expressed fusion proteins are generally not toxic to cells.
Importantly, detection does not require fixation or permeabilization of cells; therefore, compareed with immnuocytochemistry techniques using fixed cells, the likelihood of artifacts is reduced.
Its tight, barrel-like structure protects the overall conformation of GFP protein and ensures that attachment of even large protein moieties does not affect its fluorescence capacity.
Disadvantage of GFP:
It is necessary that each fusion protein be tested for its functionality in vivo because GFP-tag is so relatively large that affect the function of fused protein of interest.
The GFP signal can not be amplified in a controlled manner, possibility preventing detection of low expression levels.
In addition, folding of GFP into its active, fluorescent form is fairly slow and occurs in the order of hours. This makes the study of the fast transcriptional activation processes difficult.
|