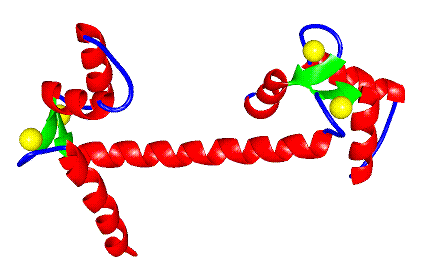
Figure 1 A ribbon diagram of X-ray crystal structure of Ca2+ calmodulin. The TR1C domain is on the left and the TR2C domain on the right.
Calcium-binding proteins play a large number of important roles in cell
metabolism. Calmodulin, in particular, has been shown to be a particularly
ubiquitous member of this family of proteins and is integral in the regulation
of the activity of a number of proteins including kinases and calcium pumps.
X-ray crystallographic studies have shown that it is a
predominantly alpha-helical protein composed of four calcium-binding
helix-loop-helix subdomains which are known as EF-hands. These EF-hands are
arranged as two per domain, with the two domains being separated by a helical
tether.

The mode of activation of calmodulin by calcium, however, remains to be definitively shown. Model studies of apo-calmodulin, based on comparison to the X-ray crystal structure of troponin C, another EF-hand type protein, have predicted that the hydrophobic patches of the two domains are concealed in the apo form by closure of the EF-hands. In order to address this issue directly, we have engaged in a study of the carboxy-terminal domain of calmodulin, TR2C. The aim of this study is to use heteronuclear multidimensional NMR to determine the structure and the dynamic behavior of TR2C in the apo and calcium-bound forms. In this way, we hope to gain insight into the mechanism of calcium induced activation of this family of calcium-binding proteins.