Structural Changes and Activation of Calmodulin
The rearrangements of secondary structure upon calcium binding yield an open binding site with a large solvent exposed surface area (Fig. 4). If we calculate the solvent-exposed surface area for the hydrophobic residues in the binding site, it is found to increase from 462 Å2 in the apo form to 726 Å2 in the Ca2+ activated structures, an increase of 57 %.
A notable exception to this is Phe-92 which undergoes a net decrease in exposed surface upon Ca2+ binding.
Using the structures obtained, these we can begin to deduce the mechanism for the activation of calmodulin.
Given the fact that the secondary structure is conserved but rearranged, the simplest mechanism would involve those calcium co-ordinating ligands that are at the ends of the helices. Bringing these ligands together via calcium coordination could induce a lever motion such that the opposite ends of the helices move away from one another.
A second, possibly complementary, effect is the unkinking of helix E on going from the apo to Ca2+ form.
The straightening of this helix could also serve to expose a portion of the hydrophobic target binding site. The mechanism of unkinking is certainly also related to the liganding of the calcium but can also be stabilized by the helix dipole of helix E.
Straightening of the helix would align the peptide dipole moments and induce a net negative charge at the C-terminus of helix E, which could interact favorably with the Ca2+ ion.
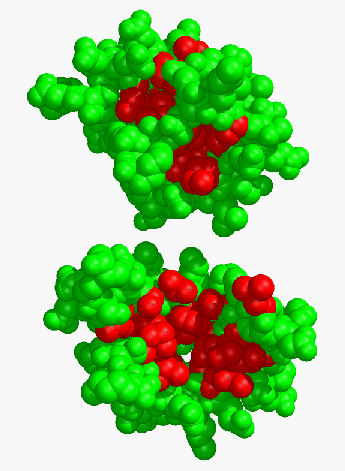
Figure 4 Space-filling models of apo (top) and Ca2+ (bottom) TR2C. Those hydrophobic residues which interact with peptide targets are indicated in red.

