2D-Gel
| 1. Sample Preparation | |||||||||||||||||||||||||||
A. Add lysis buffer to cells or IP samples. [Lysis Buffer: 9.8M Urea, 4% CHAPS (Roche), 40mM Tris-base, 1mM PMSF, 64mM DTT, 2% IPG buffer (Bio-Rad), and 1X Protease Inhibitor.] B. Incubate 30 min on nutator at RT. C. Add Rehydration Buffer to each sample (final total amount up to 300 £f). [8M urea, 2% CHAPS, few particles of Bromophenol Blue, 26 mM DTT, 2% IPG buffer (Bio-Rad)] D. Apply Samples to Rehydration Tray E. Apply the IPG Strip (pH 3-10, 17cm, Linear or NL, RioRad) to the solution containing rehydration tray. (Avoid Bubbles!!) F. Add 500£fMinerol Oil to cover the strip's surface. G. Run Rehydration program overnight. [50 volt, 50£gA/tray, run 16hrs] |
|||||||||||||||||||||||||||
| 2. IEF proforming (Isoelectric Focusing) | |||||||||||||||||||||||||||
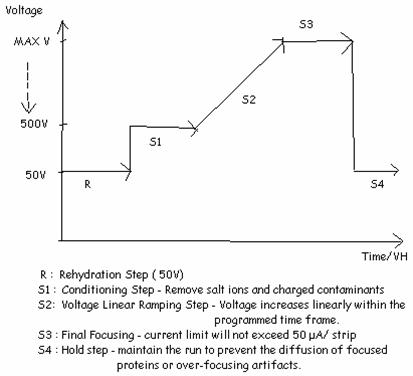
| A. Program set (total tiem ~ 9 hrs) | |||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| B. Before IEF,
move tray to the IEF focusing tray if they are nor there. Carefully remove
excess liquid from the strip with small pieces of filter paper. If the
previously covered mineral oil is not covered all the tray, add some to
fill all the surface. C. Push the "Start Button" to begin IEF focusing. D. After IEF focusing, wrap the strip, and store at -70¢J. [It could be stored for 1-2 weeks.] |
|||||||||||||||||||||||||||
|
3. Two-Dimension Separation |
|||||||||||||||||||||||||||
| A. Make 2D gels (About 20
cm X 20 cm) [Separation Gel - total amount ~ 50 ml/gel Staking Gel - total amount ~ 15-20ml/gel] B. Equilibrium Add Buf. I 3-5 ml to each strip, 15 min , RT Move strip to another tray, the add Buf. II 3-5 ml to each strip, 15 min, RT. [Buf I : 6M Urea, 2% SDS, 0.375M Tris-HCl (pH 8.8), 20% glycerol, and 130mM DTT] [Buf II : 6M Urea, 2% SDS, 0.375M Tris-HCl (pH 8.8), 20% glycerol, and 135mM Iodoacetamide] C. Rinse strips with 1-2 ml 1X running buffer. D. Place strip to the top of the 2D gel. E. Seal the strip to the gel by 0.5% Agarose in 1X running buffer. F. Load Prestained Protein Marker (about 20-25£f/gel). G. Run SDS PAGE at cold room. (60-100 mA, 5-6 hrs) |
|||||||||||||||||||||||||||
| 4. Stain with SyproRuby (Bio-Rad) | |||||||||||||||||||||||||||
| A. Wash with ddH2O, 5 min,
RT B. Fix gel with 10% Methol, 7% Acetic Acid. 30min, RT C. Replace to SyproRuby reagent. 4 hrs to O/N, RT D. Destain with 10% Methol, 7% Acetic Acid. 30min, RT E. Wash with ddH2O, 5 min, RT F. Visualize the spots under Typhoon 9410 or UV light. |
|||||||||||||||||||||||||||
| Reagents: | |||||||||||||||||||||||||||
| Lysis Buffer (1ml) | |||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Rehydration Buffer (1ml) | |||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Equilibrium Buffer I (5ml) | |||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||
| Equilibrium Buffer II (5ml) | |||||||||||||||||||||||||||
|