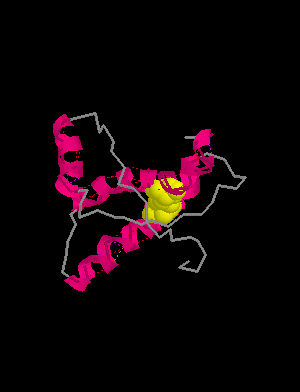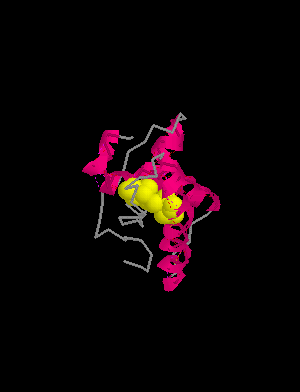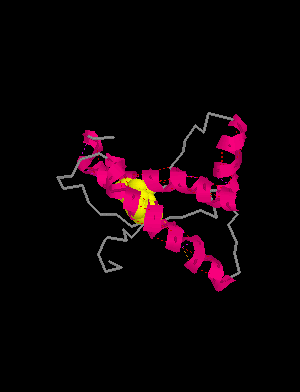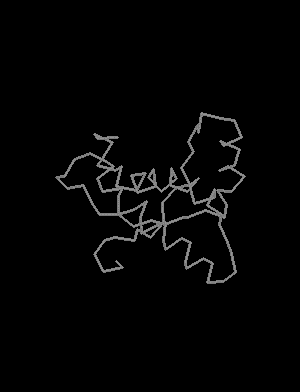
- Bovine spongiform encephalopathy (BSE) and human Creutzfeldt-Jakob disease (CJD) are among the most notable central nervous system degenerative disorders caused by prions. CJD may present as a sporadic, genetic, or infectious illness. Prions are transmissible particles that are devoid of nucleic acid and seem to be composed exclusively of a modified protein (PrPSc). The normal, cellular prion protein (PrPC) is converted into PrPSc through a posttranslational process during which it acquires a high beta-sheet content. It is thought that BSE is a result of cannibalism in which faulty industrial practices produced prion-contaminated feed for cattle. There is now considerable concern that bovine prions may have been passed to humans,resulting in a new form of CJD.
- zap
structure
HELIX 1 1 ASP 144 ASN 153 1 10
HELIX 2 2 GLN 172 LYS 194 1 23
HELIX 3 3 GLU 200 ALA 224 1 25
SHEET 1 S1 2 TYR 128 GLY 131 0SHEET 2 S1 2 VAL 161 ARG 164 -1
SSBOND 1 CYS 179 CYS 214
function
script
load "prion.pdb"
backbone on
select helix
ribbon
color structure
hbond on
select 179
select 214
script rotate
color yellow
spacefill 1.0
spacefill 1.3
spacefill 1.5
spacefill 1.7
spacefill 1.9
prion protein