It is of seminal importance to the cell that it be able to transport molecules in and out of itself. Imagine that a protein having multiple transmembrane domains is structured so that these domains are arrayed in the plane of the membrane in a circle, thereby forming a cylinder, or, better yet, a barrel when viewed from the outside of the cell, with each of the staves of the barrel being one of the transmembrane domains. The center of the barrel could constitute a hole in the plasma membrane that is isolated from the lipid bilayer by an array of transmembrane domains around it. This hole could be used to transport substances into the cell or out from the cell. In fact, this hole can be a relatively hydrophilic environment if hydrophilic side chains from the membrane-spanning chains surrounding the hole protrude into the hole itself.
In practice, given the structure of known membrane proteins, these holes are only large enough to allow the passage of small molecules through the plasma membrane, almost always simple ions like hydrogen, potassium or sodium. The ions may pass through the hole or orifice by passive diffusion, in which case the protein that allows this transport is called an ion channel. Alternatively, the transmembrane protein may invest energy, usually derived from ATP, to actively force ions from one side of the plasma membrane to the other, in which case it will be an ion pump.
Given the importance of membrane transport, cells utilize a wide range of transport mechanisms. The mechanisms fall into one of three categories: simple diffusion, facilitated diffusion, and active transport.
Simple diffusion means that the molecules can pass directly through the membrane. Diffusion is always down a concentration gradient. This limits the maximum possible concentration of the molecule inside the cell (or outside the cell if it is a waste product). The effectiveness of diffusion is also limited by the diffusion rate of the molecule (see Purves box 5.B). Therefore, though diffusion is an effective enough transport mechanism for some substances (such as H2O), the cell must utilize other mechanisms for many of its transport needs.
Facilitated diffusion utilizes membrane protein channels to allow charged molecules (which otherwise could not diffuse across the cell membrane) to freely diffuse in a nd out of the cell. These channels comes into greatest use with small ions like K+, Na+, and Cl-. The speed of facilitated transport is limited by the number of protein channels available, whereas the speed of diffusion is dependent only on the concentration gradient.

Active transport requires the expenditure of energy to transport the molecule from one side of the membrane to the other, but active transport is the only type of transport that can actually take molecules up their concentration gradient as well as down. Similarly to facilitated transport, active transport is limited by the number of protein transporters present.
We are interested in two general categories of active transport, primary and secondary. Primary active transport involves using energy (usually through ATP hydrolysis) at the membrane protein itself to cause a conformational change that results in the transport of the molecule through the protein. The most well-known example of this is the Na+-K+ pump. The Na+-K+ pump is an antiport, it transports K+ into the cell and Na+ out of the cell at the same time, with the expenditure of ATP.
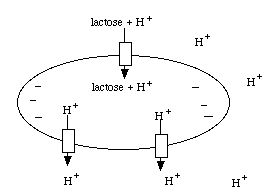
Secondary active transport involves using energy to establish a gradient across the cell membrane, and then utilizing that gradient to transport a molecule of interest up its concentration gradient. An example of this mechanism is as follows: E. coli establishes a proton (H+) gradient across the cell membrane by using energy to pump protons out of the cell. Then those protons are coupled to lactose at the lactose permease transmembrane protein. The lactose permease uses the energy of the proton moving down its concentration gradient to transport lactose into the cell. This coupled transport in the same direction across the cell membrane is known as a symport. E. coli uses similar proton driven symports to transport ribose, arabinose, and several amino acids.
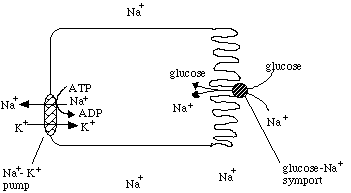
Another secondary active transport system uses the Na+-K+ pump as the first step, generating a strong Na+ gradient across the cell membrane. Then the glucose-Na+ symport protein uses that Na+ gradient to transport glucose into the cell.
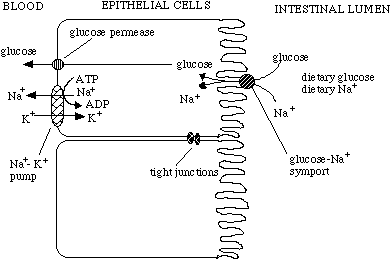
This system is used in a novel way in human gut epithelial cells. These cells take in glucose and Na+ from the intestines and transport them through to the blood stream using the concerted actions of Na+-glucose symports, glucose permeases (a glucose facilitated diffusion protein), and Na+-K+ pumps. Note that the epithelial cells are joined together by tight junctions to prevent anything from leaking through from the intestines to the blood stream without first being filtered by the epithelial cells.