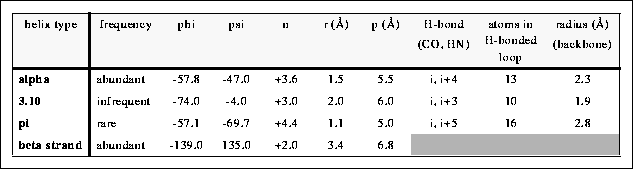
Ans: According to this table, pi helix has n=4.4 and r=16. Therefore,
pi helix can be called as 4.416
helix.
2.

The above figure shows the selected residues with statistically significant positional preferences in helices (colored regions; data taken from Richardson & Richardson, 1988). Please state the reasons that you think each residue has statistically significant preference at its corresponding position.
Ans: The helical structures require hydrogen bonds for stabalization. Yet, residues at both ends (N-terminal and C-terminal) do not have enough hydrogen bonds and they develope the "Capping Box" for extra stability.
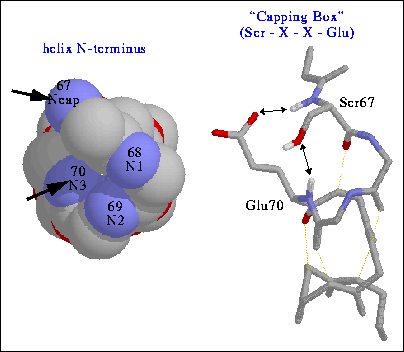
The above figure illustrates the N-capping box for N-terminal. The specific sequence (Ser-X-X-Glu) gives a stable H-bond framework. The serine amide proton and glutamine hydroxyl group form one H-bond and serine OH group forms another H-bond with glutamine amide proton.
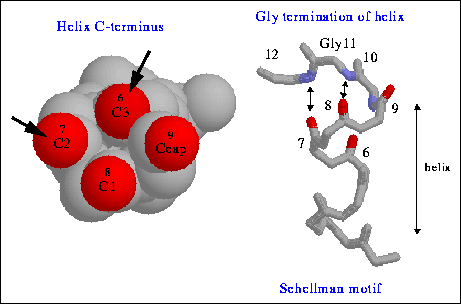
The above figure illustrate the C-capping box of the C-terminus of a helix. Like the N-capping box, the glycine at the C-terminus can form two H-bonds with its amide proton.
To explain this table, we can find, serine and glutamine are explained in the N-capping box and so does glycine in C-capping box. Asparagine has similar structure and chemical property as does glutamine. Also the acidic residues are in favored at the N-terminus because of helix has macrodiple of approximately +0.5 unit charge at N-terminus. On the other hand, basic residues such as lycine and arginine are at C-terminus because of their positive charge.
For proline, it is alway difficult to form a secondary structure from
a random coil, thus the proline provide a rigid structure to stabilize
the N-terminal structure dur its ring structure.
3. How does a prolin residue lead to the kink of a-helices?
Ans: The side chain of proline is cylic and planar when it forms a peptide
bond with other residues. The result is that the dihedral angle, psi, of
non-glycine residues proceeding prolines is restricted to positive values
(+30 < psi < +180). This (and the proline phi and psi restriction)
leads to the kink in proline-containing helices.
4. To test the role of salt bridges between oppositely charged side chains separated by three and four residues in stabilizing the a-helical conformation, two pairs of peptides of the type
were compared (S. Marqusee and R. L. Baldwin, Proc. Natl. Acad. Sci. USA 84, 8898-8902, 1987). The a -amino and a-carboxyl groups were blocked with acetyl and amide groups, respectively. How would the first and second peptides of each pair be expected to differ in their helicity? What's the reasons for these differences?AEAAAKEAAAKEAAAKAAKAAAEKAAAEKAAAEA
AEAAKAEAAKAEAAKA Blue: positively charged residues
AKAAEAKAAEAKAAEA red: negatively charged residues
5. Ion pairs in proteins involving Arg residues have been observed to be energetically stronger than those involving Lys residues. If this were the case, in what ways might Arg and Lys residues be used differently in proteins? How could this hypothesis be tested? (Hint: D. B. Wigley et al., Biochem. Biophys. Res. Commun. 149, 927-929, 1987)
Ans: Because the arginine residue forms a tighterion pair with a carboxylate
group than does lysine and these ion pairs do not break during turnover
in enzyme reactions. Thus, lysine is probably a better residue for enzymatic
reaction because of its weaker bond energy. If the binding is too tight,
it may block the casade reaction and the enzyme will die!
6.

a) This is a schematically drawing of a four-stranded beta sheet. Please indicate the antiparallel and parallel strands.
Ans: 1--->2 and 2--->3 are antiparallel beta sheets and 3--->4 is a parallel sheet.
1 ---------->
2 <----------
3 ---------->
4 ---------->
b) What is the diople moment of a b-sheet? Is it plausible that
parallel and antiparallel sheets could have substantially different dipole
interactions? (Hint: W. G. Hol et al., Nature
294, 532-536,
1981; P. T. van Duijnen et al.,
Biopolymers 24, 735-745,
1985)
Ans: The antiparallel beta sheet has zero net dipole because of the cancelation of each pair of H-bond. The parallel bete sheet has an overall macrodipole leaving the an effective charge of ~ +1/15 unit elemental charge at the N-terminus and -1/15 charge at the C-terminus of each strand of average length (~5 times less than an average alpha helix macrodipole).